Osmosis
n., plural: osmoses
[ɒzˈməʊsɪs]
Definition: net movement of water molecules across the membrane from areas of higher to lower water potential
Table of Contents
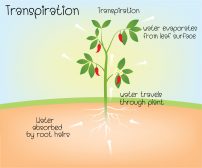
Osmosis is the net movement of solvent molecules through a semipermeable membrane. It is similar to diffusion as the movement is downhill, meaning from higher to lower concentration. In osmosis though, the movement has to occur across a semipermeable or selectively-permeable membrane. Without this element, it cannot be called osmosis.
While diffusion is often depicted as the net movement of solutes between two solutions, osmosis is about the net movement of the solvent molecules (not the solute) (solvent such as water molecules). The differing concentration of water molecules between the two sides of the membrane is what drives the water to move so as to equalize the concentrations of the two areas.
Osmosis Definition
In biology, osmosis is defined as the net movement of water molecules through a biological membrane (e.g. cell membrane) from an area of higher to an area of lower water potential. Other definitions of osmosis are as follows:
- The process of a solvent diffusing through a semipermeable membrane from an area of low solute concentration to an area of high solute concentration
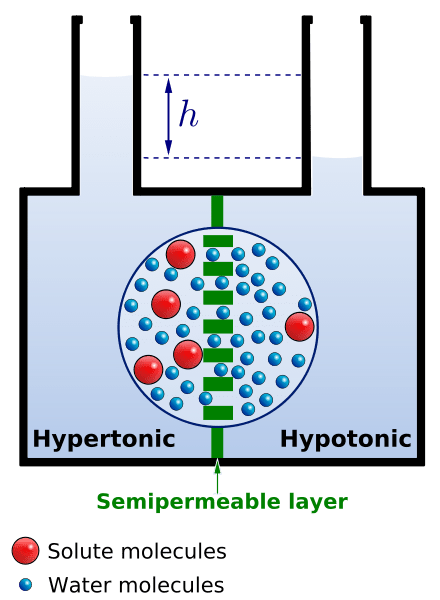
- The tendency of water to flow from a hypotonic solution (low concentration of dissolved substances) to a hypertonic solution (higher concentration of dissolved substances) across a semipermeable membrane
In chemistry, osmosis is defined similarly. It is the passage of a pure solvent from a solution of lesser to one of greater concentration of solutes when the two solutions are separated by a membrane that selectively prevents the passage of solute molecules while allowing the solvent molecules to pass through.
Etymology: The term osmosis is a Latinized form of now obsolete osmose. A derived word is osmotic, which is defined as pertaining to or of the nature of osmosis. Thus, osmotic pressure, for instance, is a pressure that arises due to or is relevant to osmosis.
Watch this vid about osmosis and water potential:
How Osmosis Works
Water molecules tend to move, and they move downhill, i.e. from an area of high water concentration (or fewer solutes) to an area of low water concentration (or greater solutes). If there is no net movement of water, it cannot be called osmosis. It should also incorporate a semipermeable membrane to move across. Without it, the process is only a case of diffusion and not osmosis.
Since water molecules are polar molecules, they need channel proteins to move down their concentration gradient. These channel proteins are embedded in the cell membrane and provide a hydrophilic passageway through which water can move across.
What drives the water molecules to move is the osmotic (pressure) gradient, i.e. differences in osmotic pressures between the two solutions. The measure of the relative tendency of water to move from one area to another is referred to as water potential. It is commonly represented by the Greek letter Ψ (Psi). Solutions that have different tonicities will cause a net flow of water across the cell membrane.
Note it!
Question: How does osmosis occur?
Answer: For osmosis to occur, these elements should be present:
- Net downhill movement of water molecules
- A selectively-permeable membrane
- Osmotic gradient
Osmotic Types of Solutions
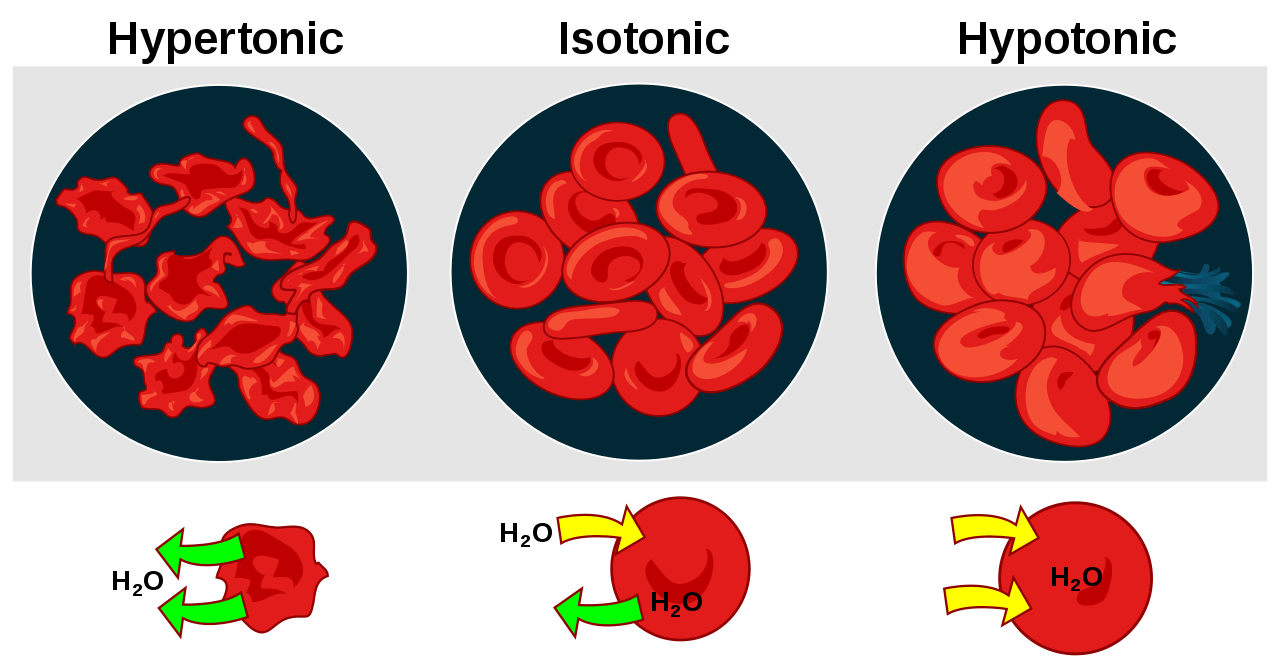
- The cell in hypertonic solution resulted in the efflux of water leaving the cell to shrink.
- In an isotonic solution, the cell apparently remains the same since the amount of water molecules leaving the cell is about the same as the amount of water entering the cell.
- In a hypotonic solution, the cell swelled from the influx of water.
A solution is comprised essentially of the solute (a substance to be dissolved) and the solvent (the component that dissolves the solutes). The concentrations of the constituents of the two solutions shall determine if a solution is isotonic, hypotonic, or hypertonic as compared to another solution.
-
Isotonic
An isotonic solution is a solution wherein the amount of solutes is basically the same as the number of solutes in another solution. For instance, a cell that is isotonic to the outside solution means that both the cell’s intracellular fluid and the surrounding fluid will have equal osmotic pressure and the same water potential. In this case, there will be no net movement of water molecules between the cell and the outside fluid.
-
Hypotonic
A hypotonic solution is a solution that has lower osmotic pressure (or has fewer solutes) than another solution to which it is compared. In this case, water moves toward the area with less water concentration or towards the more concentrated region so as to dilute the solution. For instance, when the fluid surrounding the cell is hypotonic, the water will move across the membrane toward the more concentrated solution, which is inside the cell.
-
Hypertonic
A hypertonic solution is a solution that appears to be the opposite of a hypotonic solution. A hypertonic solution will have more solutes and less water than the other solution. If a cell is immersed in hypertonic solution water will leave the cell to dilute the solution outside.
Examples of Osmosis
Here are examples of osmosis in animal and plant cells.
-
Osmosis in animal cells
In biological systems, osmosis is essential since many biological membranes are semipermeable, and it leads to different physiological effects. For example, when animal cells are exposed to a hypertonic surrounding (or lower water concentration) the water will leave the cells causing the cells to shrink. This condition is referred to as crenation. Conversely, when the animal cells are placed in a hypotonic surrounding (or higher water concentration), the water molecules will move into the cells causing them to swell. If osmosis continues and becomes excessive the cells will eventually burst.
-
Osmosis in plant cells
The cell bursting due to too much water influx does not happen in plant cells. Plants are able to counter excessive osmosis through their cell walls and vacuoles. The cell wall exerts osmotic pressure that stabilizes the plant cell. In fact, osmotic pressure is what makes plants stay upright. The large vacuole inside the plant cell also helps through osmoregulation, a regulatory process where water potential is regulated so that the osmotic pressure inside the cell is kept within the optimal range.
The plant cells, though, are not protected by water efflux. When a plant cell is placed in a hypertonic surrounding, the cell wall cannot prevent the cell from losing water. This lead to cell shrinking or the cell becoming flaccid.
Take the Osmosis Biology Quiz!
Further Reading
References
- Biology-Online Editors. (2014, May 12). Diffusion and osmosis. Retrieved from Biology-Online Dictionary Biology-Online Dictionary website: https://www.biologyonline.com/dictionary/diffusion#Diffusion-and-osmosis
- Biology-Online Editors. (2014, May 12). Turgor pressure and osmosis. Retrieved from Biology-Online Dictionary Biology-Online Dictionary website: https://www.biologyonline.com/dictionary/turgor-pressure#Turgor-pressure-and-osmosis
- Biology-Online Editors. (2014, May 12). Concentration gradient and osmosis. Retrieved from Biology-Online Dictionary Biology-Online Dictionary website: https://www.biologyonline.com/dictionary/concentration-gradient#Concentration-gradient-and-osmosis
- Biology-Online Editors. (2014, May 12). Passive transport. Retrieved from Biology-Online Dictionary Biology-Online Dictionary website: https://www.biologyonline.com/dictionary/passive-transport
© Biology Online. Content provided and moderated by Biology Online Editors