Metabolism
n., plural: metabolisms
[mɪˈtæbəˌlɪzəm]
Definition: catabolic and anabolic processes
Table of Contents
Metabolism Definition
What is metabolism in the body? Metabolism encompasses the various biochemical processes, reactions, and conversions that transform one form of energy to another. Any molecule that’s synthesized or utilized in metabolism is a physically-recognizable form of energy. The basic law of conservation of energy in Physics states that “energy can neither be created nor be destroyed; it can only be transformed from one form to another”. Following this basic rule, we can explain that biologically-active chemical molecules, too, can’t be destroyed. It can only be transformed from one physical form to another. (Feynman, 1970). And the processes that endow this molecule with the capabilities of “form transformation” are studied under “Energy Metabolism”!!!
In Biology, the definition of metabolism goes by “life-sustaining chemical reactions involving biologically-active chemical compounds and molecules”.
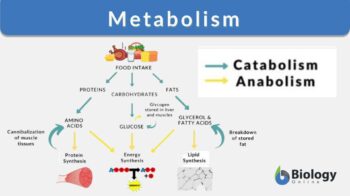
So when asked what is metabolism in biology, we can explain that it’s the biological way to conserve energy in some or the other form when different types of organisms produce or metabolize biologically-active chemical molecules.
Metabolism is the process involving a set of chemical reactions that modifies a molecule into another to essentially maintain the living state of a cell or an organism. It includes all the chemical reactions involved in modifying a molecule into another. The major functions of metabolism are storage (i.e. converting certain molecules as an energy source for various cellular processes), transforming certain molecules as a component of biomolecules (e.g. carbohydrates, proteins, lipids, and nucleic acids), and eliminating byproducts such as nitrogenous wastes.
Etymology: Greek metabolē (“change”), from metaballein (“to change”), meta- + ballein (“to throw”).
See also: anabolism, catabolism
Key Biochemicals
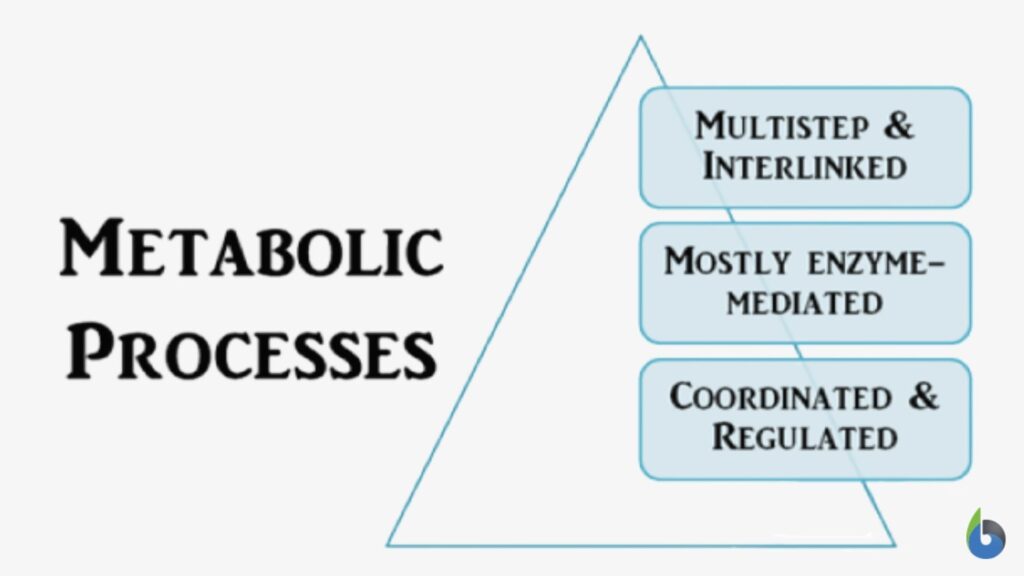
When we refer to biologically-active molecules, we are actually referring to chemical molecules that have biological activity and play a pivotal role in sustaining essential biological pathways. There are majorly 4 basic biochemicals: carbohydrates, lipids, nucleic acids, and proteins. Apart from these four are two more biochemicals that are generally studied. They are coenzymes and minerals. All these molecules play some of the most vital roles without which the proper functioning, coordination, and efficiency of biological systems.
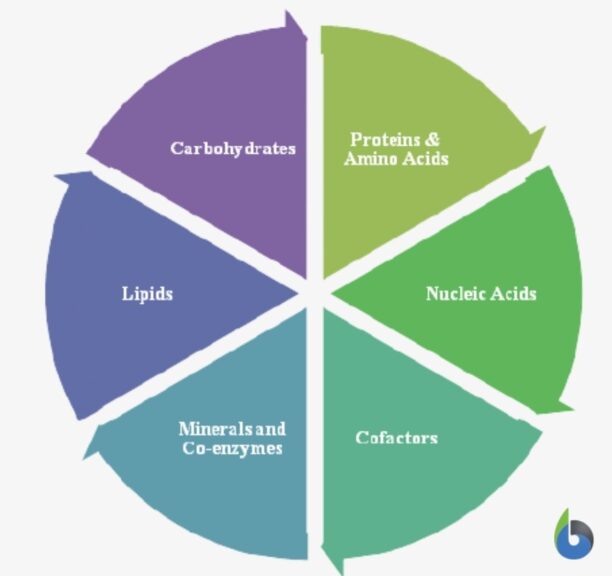
Now let’s learn about each of them in more detail and gain some useful insights about their roles and purposes in metabolic activities. Before that, we should just know these basic things:
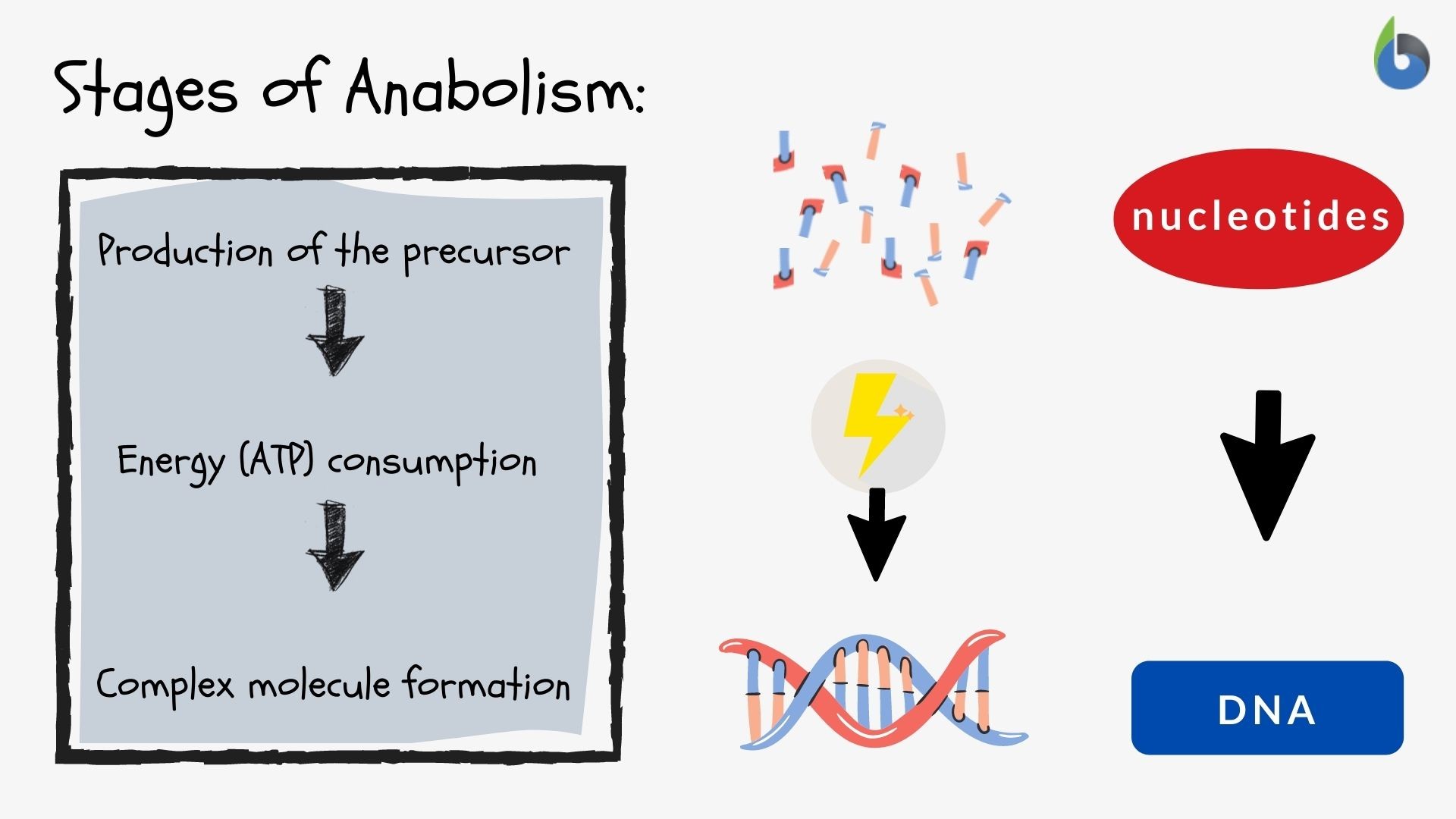
Anabolism definition: It’s the constructive metabolism accompanying synthesis and production of complex molecules from simpler monomers of biochemicals.
Catabolism definition: It’s the destructive metabolism accompanying the breakdown and degradation of complex molecules to simpler monomers of biochemicals.
1) Amino acids and proteins
What are amino acids and proteins?
Amino acids and proteins are the basic structural unit of all cells. Proteins are the building blocks of any biological entity. Proteins are actually polymers that are made up of monomers called amino acids. Amino acids are organic compounds having 2 essential groups: amino group and carboxylate groups. Then there’s one side chain group that is specific to each amino acid. Different or same amino acids are linked to each other via peptide bonds and form long peptides (polypeptides/ proteins).
Metabolism of proteins and amino acids
1. Protein Anabolism (Synthesis)
- Let’s define anabolism involving proteins! Protein anabolism is the process by which various proteins are synthesized inside/outside a biological body.
- It encompasses 2 processes: amino acid synthesis and protein synthesis (or polypeptide synthesis)
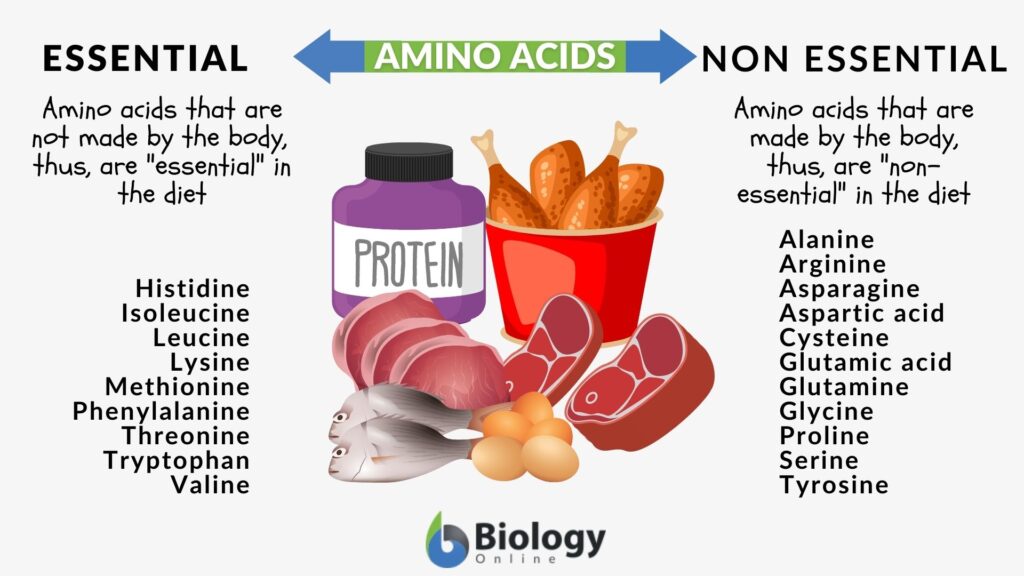
- Amino acids are of 2 types: essential and non-essential in humans. Non-essential ones can be synthesized by the human body but essential ones need to be taken via diet. There is no such concept of essential and non-essential amino acids for plants since they can produce all of them.
- Protein synthesis from amino acids encompasses 4 major steps: transcription, translation of proteins, PTMs (post-translational modifications), and protein folding
2. Protein Catabolism (Breakdown)
- The breakdown of proteins is specifically called proteolysis.
- After the proteins are broken down to the monomeric form i.e. amino acids, they are further reduced by degradations to individual atoms like nitrogen, oxygen, carbon, and hydrogen (also sulfur, selenium in some specific amino acids).
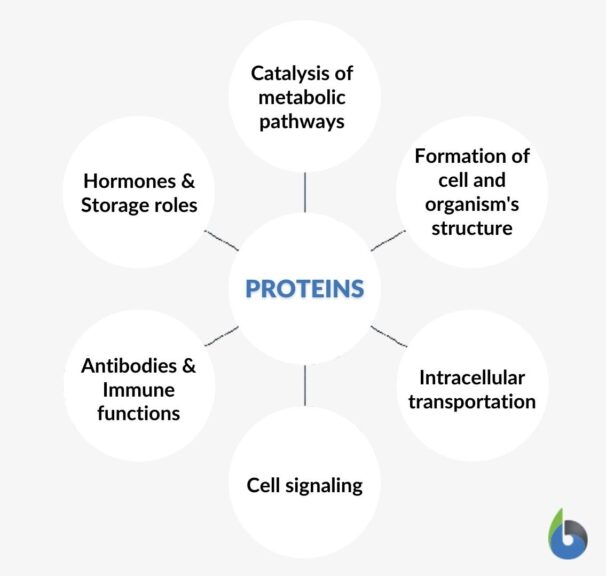
Vital roles performed by proteins are:
- Catalysis of metabolic pathways
- Formation of cell and organism’s structure
- Intracellular transportation
- Cell signaling
- Antibodies and immune functions
- Hormones and storage
2) Lipids
What are lipids?
Lipids are the biochemicals that don’t dissolve in polar solvents but only in non-polar solvents. Most of the lipids are either amphipathic or hydrophobic. Amphipathic, literally means a molecule that has both hydrophilic and hydrophobic parts. There’s an entire array of lipids in the biological world ranging from simple fats to PUFAs (polyunsaturated fatty acids), from mono- & triglycerides to long-chain prenol lipids, from different types of phospholipids and sphingolipids to sterols.
While some lipids are non-essential for animals and mammals as they can be derived from cetain lipids in the body, other lipids like ALA (alpha-linolenic acid) and LA (linoleic acid) are essential for the human body. There’s no such concept of essential and non-essential lipids for plants; they are the major producers of lipids on this planet.
- Site of major lipid and fat metabolism in animals: liver, pancreas
- Site of major lipid metabolism in plants: plastids and peroxisomes
Metabolism of lipids
1. Lipids Anabolism (Synthesis)
- Lipid anabolism is the process by which lipids are synthesized biologically.
- Plants are the major producers of lipids on the planet.
- Plants and bacterial systems are characteristically different from animals and mammals in lipid production since they possess distinct enzymes for each step of lipid biogenesis while the animals and mammals possess a multi-functional enzyme that carries out all the steps of lipid synthesis.
- Plants possess special desaturase enzymes that aid in the introduction of double bonds after carbon 9 and 10. Mammals are devoid of any such enzyme and thus can’t synthesize their own omega fatty acids (omega-3 and omega-6); making these the “essential fatty acids” that need to be obtained from the diet.

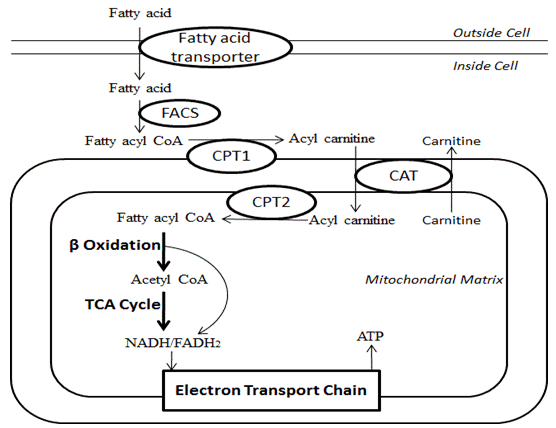
2. Lipids Catabolism (Breakdown)
- The process of lipid breakdown is called beta-oxidation.
- Sites of beta-oxidation in plants: peroxisomes and glyoxysomes
- Sites of beta-oxidation in animals and mammals: mitochondria and peroxisomes
Vital roles performed by lipids are:
- Storage of energy
- Structural component of membranes
- Hormonal and homeostatic functions
- Signaling and chemical messenger
- “Cushion” of vital organs
- Transport of fat-soluble nutrients
Some essential roles performed by lipids are depicted in the figure below. Ranging from various biological metabolic functions of membrane composition to energy storage and from various cell signaling roles to hormonal and behavioral functions, lipids contribute to the basic and intricate functioning of a metabolic living cell.

3) Carbohydrates
What are carbohydrates?
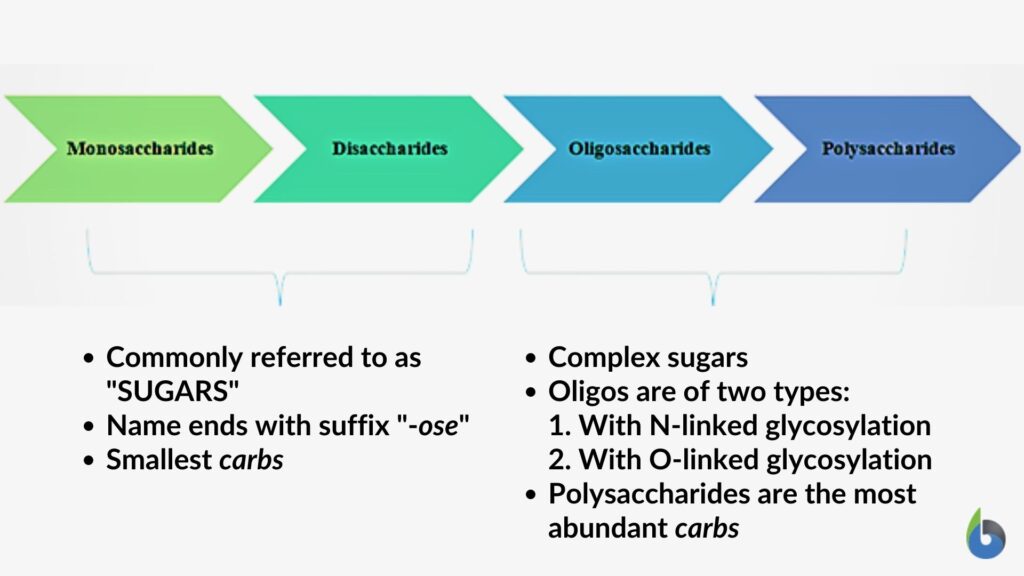
Carbohydrates are basically the hydrates of carbon; mainly consisting of carbon, oxygen, and hydrogen atoms. The four main types are mono-, di-, oligo– and poly-saccharides.
- Monosaccharides: The main fuel source of all biological activities is monosaccharides. Glucose, ribose, fructose, trehalose, ribulose, xylulose, galactose, mannose, deoxyribose, and lyxose are examples of monosaccharides and serve as important precursors of many metabolic activities inside a cell.
- Disaccharides: Disaccharides are also simple carbohydrates that are formed via the joining of two monosaccharides by glycosidic linkages. Lactose, maltose, sucrose, and cellobiose are some examples of disaccharides.
- Oligosaccharides: Oligosaccharides are small polymers of sugars; of about 3-10 monosaccharides joined together. Raffinose series, maltodextrin, cellodextrin are some examples of oligosaccharides.
- Polysaccharides: Polysaccharides are polymeric complexes that are composed of 200-2500 monosaccharides joined together by glycosidic linkages. They are both linear and branched. Starch, cellulose, chitin, glycogen, and galactogen are some examples of polysaccharides.
Metabolism of carbohydrates
1. Carbohydrates Anabolism (Synthesis)
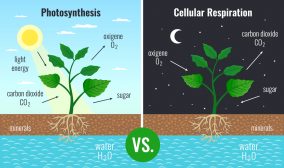
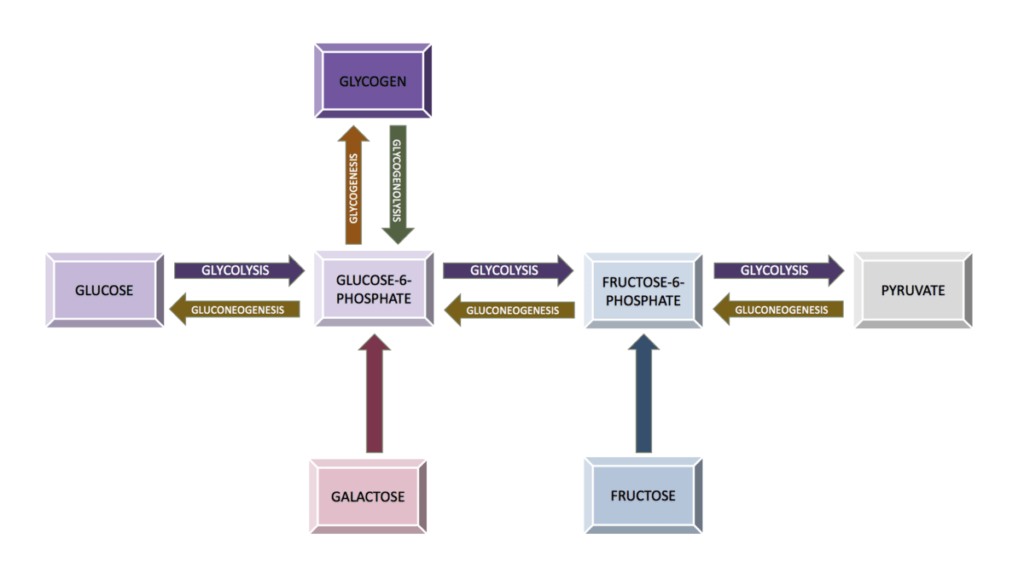
- Plants: Plants synthesize their own carbohydrates via photosynthesis from carbon dioxide, sunlight, and water.
- Animals & Fungi: Gluconeogenesis is an anabolic reaction method of carbohydrate production from non-carbohydrate sources in animals and mammals. The site of gluconeogenesis is the liver in vertebrates. There is another method of carbohydrate synthesis called glycogenesis; the process of conversion of glucose to glycogen.
2. Carbohydrates Catabolism (Breakdown)
- The breakdown of carbohydrates like glucose happens via glycolysis. Glycolysis happens in both plants and animals.
- The breakdown of carbohydrates like glycogen happens via glycogenolysis, a catabolic reaction process.
Vital roles performed by carbohydrates are:
- Storage of energy
- Structural components
- Coenzyme component
- Nucleotide component
- Role in the immune system, fertilization
- Role in growth and development

4) Nucleotides
What are nucleotides?
Nucleotides are the basic building structural and functional blocks of nucleic acids.
- Nitrogenous Base + Sugar → Nucleoside
- Nucleoside + 1 Phosphate group → Nucleotide Monophosphate
- Nucleotide Monophosphate + 1 Phosphate group → Nucleotide Diphosphate
- Nucleotide Diphosphate + 1 Phosphate group → Nucleotide Triphosphate (Nucleic Acids)
The different types of nucleic acids and the nucleotide nomenclature can be understood from the table below:
Table 1: Nucleotide and nucleic acid nomenclature |
|||
|---|---|---|---|
| Base | Nucleoside | Nucleotide | Nucleic acid |
| Purines | |||
| Adenine | Adenosine
Deoxyadenosine |
Adenylate
Deoxyadenylate |
RNA
DNA |
| Guanine | Guanosine
Deoxyguanosine |
Guanylate
Deoxyguanylate |
RNA
DNA |
| Pyrimidines | |||
| Cytosine | Cytidine
Deoxycytidine |
Cytidylate
Deoxycytidylate |
RNA
DNA |
| Thymine | Thymidine or
Deoxythimidine |
Thymidylate or
Deoxythymidylate |
RNA
DNA |
| Uracil | Uridine | Uridylate | RNA |
| Data Source: Bioinfo.org – Biochemistry | |||
Metabolism of nucleotides
1. Nucleotides Anabolism (Synthesis)
- All living cells are capable of producing nucleic acids.
- Dietary intake of nucleic acids isn’t really needed.
- Nucleic acids from the diet are actually degraded and reverted back for some more anabolic-type metabolic reactions via salvage pathways.
2. Nucleotides Catabolism (Breakdown)
- Catabolism of nucleotides and nucleic acids is a continuous process that keeps going on as the nucleotide anabolism.
- There are only two possible end destinations of nucleotides: one is degradation into waste product and final excretion, second is the re-entry of catabolized products via salvage pathway into nucleotide production.

Vital roles performed by nucleotides are:
- Storage and transfer of genetic information
- Co-substrates and coenzymes
- Cellular signaling
- Source of a phosphate group
Nucleotides perform some vital biological functions inside a living cell.

5) Coenzymes
Coenzymes are very small molecules that themselves can’t catalyze biological reactions; the binding with apoenzymes makes holoenzymes. Holoenzymes are the active state of enzymes while apoenzymes are the inactive or less active state. Many different vitamins actually serve as coenzymes.
Table 2: List of various coenzymes and details about their specificities. |
|||
|---|---|---|---|
| Coenzyme | Vitamin from which it’s derived | Functional group or atom/s transferred | Example of dependent enzyme |
| TPP (Thiamine Phosphate) | Thiamine (B1) | Aldehyde | Transketolase |
| FMN (Flavin Mono Nucleotide) | Riboflavin (B2) | Hydrogen & Electrons | L-amino acid oxidase |
| FAD (Flavin Adenine Dinucleotide) | Riboflavin (B2) | Hydrogen & Electrons | D-amino acid oxidase |
| NAD (Nicotinamide Adenine Dinucleotide) or DPN (Diphospho Pyridine Nucleotide) | Niacin (B3) | Hydrogen & Electrons | Lactate Dehydrogenase |
| Coenzyme A | Pantothenic Acid (B5) | Acyl | Thiokinase |
| PLP (Pyridoxal Phosphate) | Pyridoxine (B6) | Amino | Alanine Transaminase |
| Biotin | Biotin (B7) | CO2 | Pyruvate Carboxylase |
| Tetrahydrofolate | Folic Acid | One Carbon Unit | |
6) Minerals & Cofactors
Minerals and cofactors are some essential biochemicals in living bodies. Cofactors can be any of the two: organic or inorganic. They aid in the functioning of the enzymes.
- Cofactors are different from coenzymes as cofactors are in general chemical compounds while coenzymes are a type of cofactors that are biological molecules.
- On one side while cofactors are inorganic in nature, coenzymes are organic in nature.
- Cofactors are tightly/covalently bound to the enzymes while contrastingly the coenzymes are loosely bound to enzymes.
- Cofactor for enzymes examples: Metal ions Mg2+, Cu+, Mn2+
How Does Metabolism Work?
Metabolism works by the balance of anabolic and catabolic activities. Any metabolic activity that a living body performs requires some energy. For any such energy, there has to be a source of energy. Where does energy come from? Now, recall the law of Physics cited at the beginning of the article, which states that energy can neither be created nor be destroyed; it can only be transformed from one form to another. So the energy that’s needed for any work to be performed by a living body is derived from a storage source of energy, which is usually carbohydrate or lipid.
The next question is “how do such sources form in the first place?”
The answer to this is during the basic biological growth and development of a living being, it tends to either produce its own sources of energy by using other sources. For instance, plants use energy from sunlight to produce their own sources of food (energy). While on the other hand, animals and fungi depend on these photosynthetic organisms or other animals for their energy source.
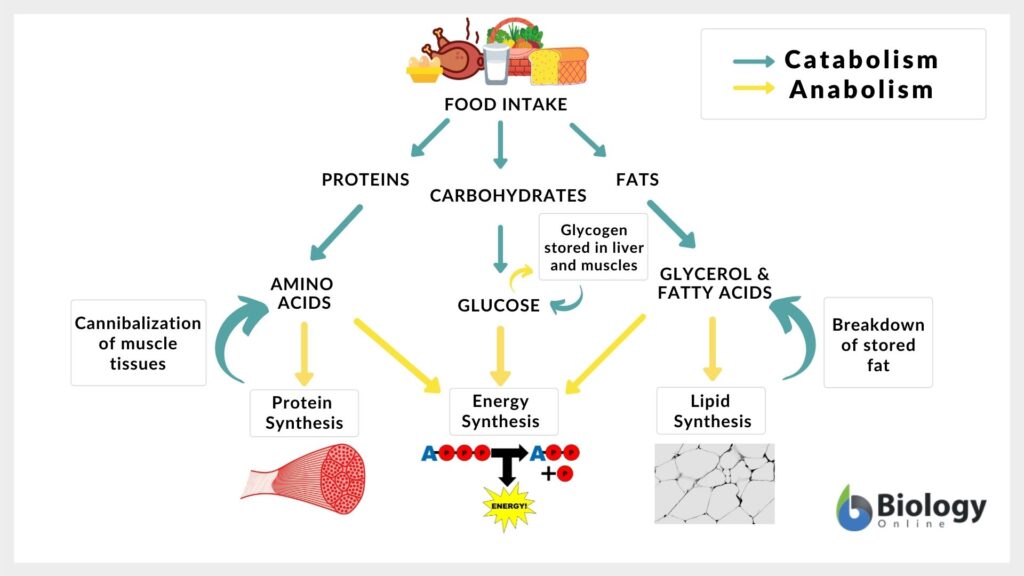
Types of Metabolism
There are basically 2 types of metabolism that must be very clear by now: anabolism (synthesis) and catabolism (degradation). Look at the table below to learn some major differences between the two types of metabolism.
Table 3: Difference between anabolism and catabolism |
||
|---|---|---|
| Criteria | Anabolism | Catabolism |
| Process | Synthesis process (Constructive) | Breakdown process (Destructive) |
| ATP requirement | Required | Released |
| Energy-wise | Endergonic | Exergonic |
| Oxygen utilization | No | Yes |
| Important role in | Growth and development, bone mineralization, etc | Digestion, respiration, etc. |
When asked what are the 3 types of metabolism in the human body. We can explain endomorph, ectomorph, and mesomorph. Endomorphs have the slow metabolism amongst all while ectomorphs have the most active and the fastest metabolism. Mesomorphs have the perfect metabolism, which is a balanced one between endomorph (low metabolism) and ectomorph (fast metabolism).
Energy Transformations
Since the biological diversity is very wide, the types of energy transformations one can witness are also wide. On one hand, we see some organisms deriving their energy from light, while on the other hand, we see some others deriving their energy from minerals and chemicals. On one hand, we see some organisms synthesizing and storing their own food as storage of energy for future purposes, at the same time we see some others depending on the former ones for deriving their nutrition.
Oxidative phosphorylation
- Happens in both prokaryotes and eukaryotes.
- Mainly constitutes two steps: electron removal and ATP derivation (for energy)
- Location in eukaryotes: Mitochondrial membrane (in ETC or electron transport chain)
- Location in prokaryotes: In the inner membrane of cells
- Also called electron transport-linked phosphorylation

Energy from inorganic compounds
- This type of metabolism is present in prokaryotes.
- Called “chemolithotrophy”
- Major role in biogeochemical cycles as these types of energy derivations aid in maintaining the flow of electrons, energy, and atoms from one medium to another. This also aids in soil fertility.
Energy from light
- This type of metabolism is present in green plants, algae, some bacteria, and some protists.
- It helps in photosynthesis in plants and algae.
- It helps in easy switching between two different modes of metabolism in bacterial and protist systems.
Xenobiotics and Redox Metabolism
Xenobiotics are those chemicals and molecules which if not properly managed inside a cell can cause immense harm to a biological system. So, a proper system is vital to manage, dispose and clear these harmful compounds in living beings. Humans possess some specialized enzymes that metabolize xenobiotics like:
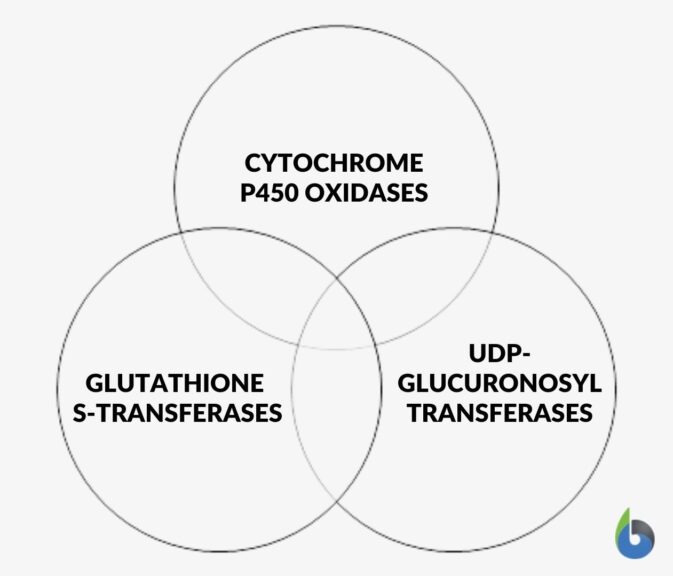
Thermodynamics of Living Organisms
It is expected out of living organisms and bodies that they will obey the basic laws of thermodynamics. The law of entropy states that in any closed system, entropy should always increase and “never” decrease. Eventually, this leads to an increase in metabolism in biological things. But as we know that all living organisms dissipate energy and are open systems, the laws of thermodynamics aren’t really challenged much!
Regulation and Control
Regulations and control are important for a variety of reasons:
1) Helps in maintaining “homeostasis”- the constant basal metabolism of a body
2) Helps in proper signal management and interaction of an organism with its environment.
Control is of 2 ways:
1) Intrinsic Control– via intrinsic factors, feedback controls, allosteric regulatory systems
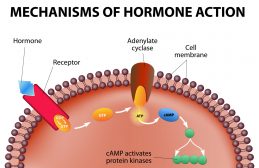
2) Extrinsic Control– via growth factors, hormones, secondary messenger systems, and protein phosphorylation steps
Evolution
Why do organisms and their metabolic processes evolve? The simple answer to this is that evolution always aims at the optimization of beings and processes. Hence, when organisms evolve, simultaneously the basic life processes also evolve. But when we look down the evolutionary timescale, we notice one very important thing that these basic metabolic processes of different biochemicals like carbohydrates, lipids, proteins, and nucleic acids didn’t change much down the timelines. This point towards an important finding. “All these metabolic processes have already been optimized via evolution so much that now all 3 domains of life share the same basics.” Additionally, the last ancestors that we notice also shared the same metabolic processes, pathways, and steps.
Investigation and manipulation
- To study the entire array of metabolic pathways and products, a special field has been defined now. It’s called Metabolome!
- Radioactive probes are used to trace the entire pathway and study the steps of importance.
- It has a role in metabolic engineering and aids in advancing our efforts for human welfare by playing with the biological model systems.
Conclusion
By now, you would have likely gained a lot of clarity about what metabolism means, how different variety of living organisms come on the same ground when metabolic processes are compared, why metabolism is so important in biology, what metabolism does across the variety of body tissues, examples of metabolism, and sites and functions of each pathway.
Try to answer the quiz below to check what you have learned so far about metabolism.
Further Reading
- Plant Metabolism – Biology Online Tutorial
- Protein Activity and Cellular Metabolism – Biology Online Tutorial
References
- Richard Feynman (1970). The Feynman Lectures on Physics Vol I. Addison Wesley. ISBN 978-0-201-02115-8.
- Harwood, J.L. (2005).Fatty acid biosynthesis. In: Plant Lipids: Biology, Utilisation and Manipulation, pp. 27-66 (D.J. Murphy (ed.), Blackwell Publishing, Oxford)
- Kim H.U. (2020) Lipid Metabolism in Plants. Plants. 9(871) DOI:10.3390/plants9070871
- Prof. Y. Waisel, A. Radunz. (2006) Differences in lipid and in fatty acid composition of taproots and lateral roots of faba bean plants (Vicia faba L.) grown in saline media. Plant Biosystems – An International Journal Dealing with all Aspects of Plant Biology 140:1, pages 94-99.
- Mogens Kilstrup, Karin Hammer, Peter Ruhdal Jensen, Jan Martinussen, Nucleotide metabolism and its control in lactic acid bacteria, FEMS Microbiology Reviews, Volume 29, Issue 3, August 2005, Pages 555–590, https://doi.org/10.1016/j.fmrre.2005.04.006
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.