Alanine
n., plural: alanines
[ˈæl əˌnin]
Definition: an amino acid with a formula, C3H7NO2
Table of Contents
What is alanine? Alanine is a non-essential amino acid. There are two types of amino acids for mammals namely essential and non-essential amino acids.
Essential amino acids are the indispensable amino acids that need to be supplied by the means of diet whereas non-essential amino acids are produced by the body and hence not required via diet.
Look at the table below to learn the major differences between the two types.
Table 1: Differences between essential and non-essential amino acids |
||
|---|---|---|
| Characteristic Feature | Essential Amino Acid | Non-essential Amino Acid |
| Synthesis by the human body | No | Yes |
| Required via diet | Yes | Not necessarily |
| Deficiency due to | Non-availability of these amino acids in the diet | Non-availability of the precursors and enzymes needed for their synthesis |
| Alternative name | Indispensable amino acids | Dispensable amino acids |
| Number in the human body | 9 for adults, 10 for infants (one additional arginine) |
11 for adults, 10 for infants (one less i.e. arginine) |
| Can they become opposite in some conditions? | Absolutely NO (essential a.a. can never become non-essential a.a.) |
Conditionally YES (non-essential a.a. can become essential when the necessary precursor/s or enzymes are missing.) |
| Examples | Histidine, methionine, isoleucine, threonine, leucine, valine, lysine, tryptophan, and phenylalanine. | Proline, glycine, alanine, cysteine, asparagine, glutamine, tyrosine, serine, arginine, aspartic acid, and glutamic acid. |
Note: Infants aren’t capable of producing enough arginine to meet their bodies’ needs; hence arginine needs to be provided in their diets. Data Source: Akanksha Saxena of Biology Online
Alanine Definition

Alanine is an aliphatic, non-polar, non-aromatic, non-essential, crystalline α-amino acid that is synthesized by the human body using precursors and enzymes. It is one of the simplest amino acids with a methyl group as its side chain. It is the second simplest amino acid after glycine in which a hydrogen atom is the side chain.
The symbol for alanine is A and the 3-letter code is Ala. The IUPAC name of alanine is 2-aminopropanoic acid. It serves several roles ranging from its involvement in the energy-producing breakdown of glucose, biosynthesis of protein structure, carrier molecule in anaerobic respiration, etc. Since the body usually synthesizes enough alanine, deficiency syndromes pertaining to alanine amino acids are uncommon.
History and Etymology
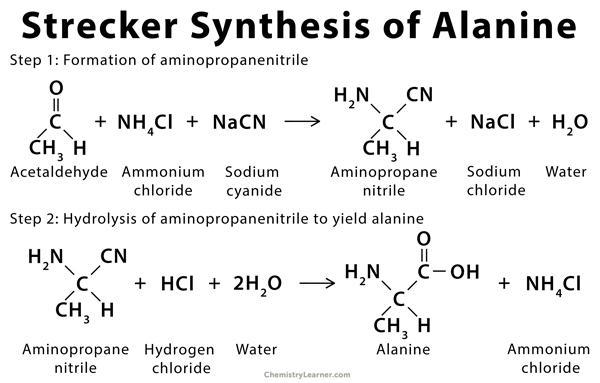
- History: This amino acid was first synthesized by Adolph Strecker in 1850. Alanine was synthesized by combining acetaldehyde, ammonia, and hydrogen cyanide.
- Coining of the term: The term alanine was named as ‘alanin’. It was later changed to ‘alanine’.
- Etymology: The word ‘alanin’ is German in its origin, referring to ‘aldehyde’. And –in is used in German language for chemical compounds like –ine is used in the English language.
Biology definition:
Alanine is an aliphatic, non-polar, non-aromatic, non-essential, crystalline α-amino acid that is synthesized by the human body. The IUPAC name of alanine is 2-aminopropanoic acid with symbol ‘A’ and 3-letter code ‘Ala’.
Alanine Structure
Let’s understand the structure of alanine and learn what type of amino acid is alanine?
- Alanine is an aliphatic amino acid, i.e. the hydrocarbon chain is a straight or branched chain amino acids or without an aromatic ring structure.
- Alanine is a non-polar amino acid, i.e. the functional group is uncharged at the physiological pH (at physiological pH, alanine charge=0). Additionally, it can’t participate in any hydrogen bonding at physiological pH.
- Alanine is a non-aromatic amino acid, i.e. there is no aromatic ring structure. This makes it clear why alanine doesn’t absorb UV light at 250 nm and hence doesn’t display fluorescence.
- Alanine is a non-essential amino acid, i.e. the body’s requirement of alanine is met by the body’s production of alanine. There is no need for dietary intake of alanine in normal individuals.
- Side chain of alanine: Methyl group (-CH3).
- Nature of side chain: Non-reactive (hence, rarely observed to form hydrogen bonds or involvement in protein function)
- Major structural components of alanine: an amine group, a carboxylic acid group, and a methyl group (side chain) – all three groups being attached to a central α-carbon atom.
- Form under biological conditions: Zwitterionic form (Amine group is ‘protonated’ forming a −NH3+) while the carboxyl group is ‘deprotonated’ forming a –CO2–).
- Codons encoding alanine: codons start with GC, i.e., GCA, GCU, GCG, GCC.
- Isomers of Alanine: There are two isomers of alanine, L-isomer (left-handed) and D-isomer (right-handed).
- L-isomer: Isomer that’s incorporated into proteins. The rate of occurrence of L-alanine is quite high in the primary structure of proteins (~7.8%), i.e., proteinogenic amino acids. In general, it is the 2nd highest of all the amino acids in almost all the proteins (1st=Leucine).
- D-isomer: This is the uncommon isomer in eukaryotic life forms. Its presence has been noted in some polypeptides of the cell walls of prokaryotic life forms like bacteria (like E. coli). Its presence has also been noted in some peptide antibiotics. It also constitutes some proteins in the tissues of crustaceans and mollusks.
Sources
Although the human body is capable of synthesizing its own alanine, there are also some external sources of alanine from which it can be taken in case of deficiency. Some of these sources are (1) chemical synthesis and (2) biological sources: Meat, poultry, fish, eggs, tofu, dairy products like cheese, etc.
-
Biosynthesis
The biological synthesis of alanine happens by the integration of pyruvate and some source of branched-chain amino acids like isoleucine, valine, or leucine. It is a two-step process. Pyruvate undergoes ‘reductive amination’.

-
- Step-1: Production of Glutamate – A molecule of α-ketoglutarate combines with a molecule of ammonia and NADH to give a glutamate molecule, NAD+ and a water molecule. The enzyme involved in step 1 is glutamate dehydrogenase.
α-ketoglutarate + Ammonia + NADH 🡪 Glutamate + NAD+ + Water - Step-2: Transamination Reaction – The –NH2 group of the newly formed glutamate is transferred to the pyruvate molecule. (amino donor=glutamate) This changes the pyruvate into an ‘alanine molecule’. In this process, a molecule of ‘α-ketoglutarate’ is regenerated. The enzyme involved in step 2 is aminotransferase/transaminase.
- Step-1: Production of Glutamate – A molecule of α-ketoglutarate combines with a molecule of ammonia and NADH to give a glutamate molecule, NAD+ and a water molecule. The enzyme involved in step 1 is glutamate dehydrogenase.
-
Chemical synthesis

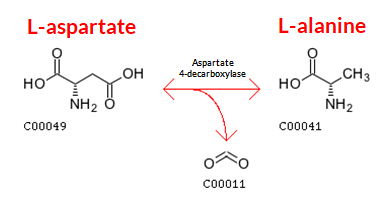
Figure 4: Production of L-alanine is performed by decarboxylation of L-aspartate. Image Source: Akanksha Saxena of Biology Online - Production of L-alanine: Industrial and commercial production of L-alanine is done chemically by decarboxylating L-aspartate. This is performed with the help of an enzyme called ‘aspartate 4-decarboxylase’. Alanine racemase is frequently used to complicate the fermentation routes that lead to the production of L-alanine.
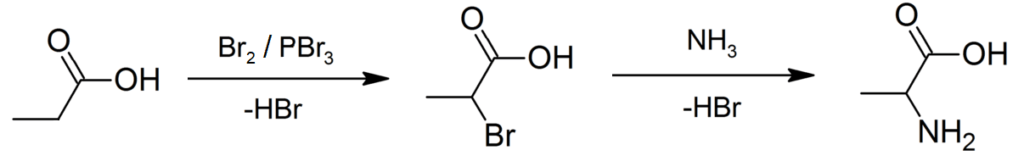
- Production of Racemic alanine: It is done by the Strecker reaction (condensation reaction of acetaldehyde and ammonium chloride in sodium cyanide’s presence). It can also be done by ammono-lysis of 2-bromopropanoic acid (BPA).

Figure 5: Production of racemic alanine via direct ammono-lysis of 2-bromopropanoic acid. Image Credit: Rifleman 82, EdChem.
-
Degradation
- Process of alanine degradation: Oxidative deamination
- Enzyme responsible: Aminotransferase/transaminase and PDC (pyruvate dehydrogenase complex)
- Oxidative deamination is the inverse of reductive amination that is used to synthesize alanine.
- As you notice, the enzymes are the same. The direction of these reversible reactions involved (synthesis and degradation) here depends on the ‘relative concentrations of both substrates and products!)
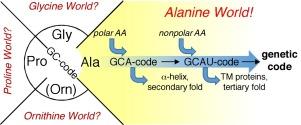
Alanine World Hypothesis
- Idea: Protein synthesis is mediated by ribosomes using different amino acids as building blocks. Alanine is one of them. The existence of alanine is estimated to be one of the longest among the different amino acids. Even its inclusion in the standard repertoire of genetic code is estimated to be very old. This prompted the proposal of the ‘alanine world hypothesis’.
- Explanation: This hypothesis aims to explain the rationale behind the evolutionary choice/s of different amino acids from a chemical point of view. The hypothesis tries to explain that this choice-making for an amino acid in the repertoire of genetic code is ‘evolutionarily-controlled and driven’. It elucidates that those specific alanine derivatives which could be suitable for specifically building ‘α-helix’ or ‘β-sheets’ like secondary structures in the protein synthesis are favored above all the other amino acids.
- Note: As we look around, we can notice the α-helices and β-sheets are dominant secondary structures of proteins in almost all life forms. Additionally, the most canonical amino acids are regarded as ‘alanine derivatives’. This is the reason that alanine can exchange the most canonical amino acids in all proteins via the mechanism of point mutations. This doesn’t even disturb the secondary structure of proteins.
Physiological Function
Since the biosynthesis of alanine depends on the presence of pyruvate which is present in almost all types of cells, it is easily and ubiquitously produced by the body. This is the reason that we find a close association of alanine to numerous metabolic pathways such as glycolysis (breakdown of glucose), gluconeogenesis (synthesis of glucose), and the citric acid cycle. The different functions of alanine are:
-
Role in anaerobic respiration
When the body suddenly switches to anaerobic metabolism, proteins of the muscle tissues undergo breakdown for deriving quick energy. Under such conditions, alanine behaves as a “carrier molecule”. It carries the N-containing amino groups from the muscles to the liver. This helps in the prevention of toxic metabolic build-up in muscles. The processing of amino groups into less toxic urea is ensured in liver cells.
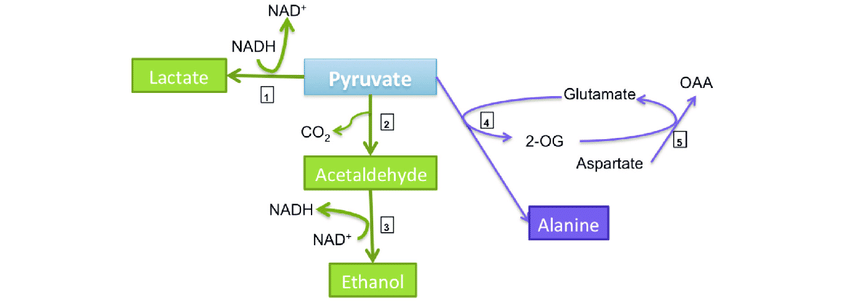
-
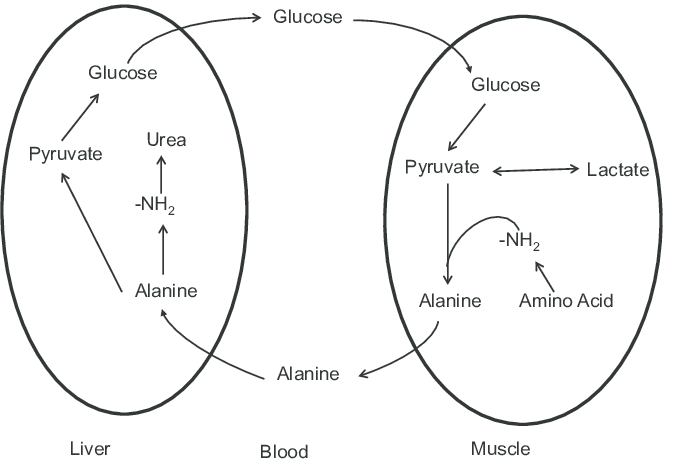
Glucose–alanine cycle
As discussed in the above section, alanine plays an indispensable role in the clearance of toxic build-up of glutamate and pyruvate. This cycle is called the glucose-alanine cycle.
Cycle operation between: Muscle tissues and liver
Steps of glucose-alanine cycle: The steps are as follows.
- Muscle breakdown comprises protein degradation.
- Degraded amino groups collection (form= glutamate, process=transamination)
- Transfer of –NH2 group from glutamate to pyruvate [(pyruvate=muscle glycolysis product), (enzyme=alanine aminotransferase), (product=alanine + α-ketoglutarate)]
- Entry of alanine into the bloodstream
- Transport of alanine to liver
- A reverse reaction of alanine aminotransferase is conducted in the liver
- Pyruvate regeneration and its usage in process of gluconeogenesis
- Transfer of newly formed glucose molecules from the liver to muscles through the bloodstream
- Entry of glutamate in mitochondria of liver cells
- Breakdown of glutamate into α-ketoglutarate and ammonium (enzyme: glutamate dehydrogenase)
- Ammonium is involved in the urea cycle and leads to urea formation
- Urea is excreted by the kidneys
Significance: This cycle ensures the removal of pyruvate and glutamate from the muscles and tissues. This cycle also ensures the regeneration of glucose. This cycle takes care of the muscle tissue by shifting the energy-generation burden from muscles to the liver. This step is to make sure that all the available ATP molecules in the muscle tissues can be solely devoted to the process of muscle contraction.
Watch this vid about glucose-alanine cycle:
-
Link to diabetes
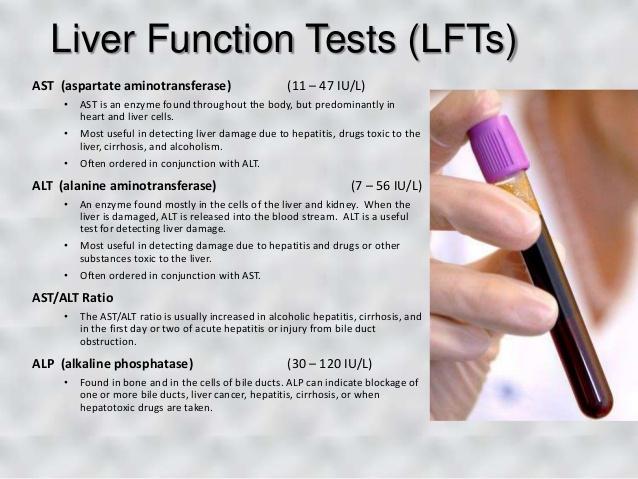
Any alterations or manipulations or malfunctions in the alanine cycle can lead to the development of type-II diabetes. This is based on the linkage between an abrupt increase in serum alanine aminotransferase levels (ALT levels) and disruption of the alanine cycle.
-
Link to liver disease
The alanine aminotransferase (ALT) test is also performed for testing for some liver diseases.
Chemical Properties
- Alanine formula: C3H7NO2
- As alanine is a straight chain amino acid, it doesn’t have an aromatic ring. Because of the lack of aromatic rings, it’s unable to absorb UV light at 250 nm and thereby lacks fluorescence. Unlike aromatic amino acids, alanine can’t be used for quantification purposes.
- Alaninol is a hydrogenation product of alanine. It’s amino alcohol and a useful chiral alanine building block.
- Alanine molar mass: 89.09 g/mol
Free Radical
- Produced by the process of deamination
- Induction of deamination process via radiation (leads to C-N bond cleavage)
- Notation of the free radical: CH3C*HCO2–.
- Uses: Useful in ‘dosimetric measurements in radiotherapy
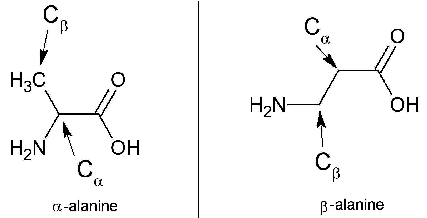
The curious case of β-Alanine
β-Alanine is the rarer form of naturally-occurring alanine. It is popularly used by athletes for their performance enhancement. β-Alanine limits the carnosine levels. Supplementation of β-Alanine directly increases muscle carnosine levels. This is explained to be inversely related to ‘the muscles fatigue in athletes’ and directly related to ‘the ability to perform total muscular work’.
This has increased the popularity of β-Alanine supplementation amongst athletes and bodybuilders. Another important point to note is that direct intake of carnosine doesn’t solve the purpose of performance enhancement as oral intake of carnosine leads to mere digestion of it to its components.
While some athletes prefer to take beta-alanine alone, some others like to design a combination like beta-alanine and creatine, beta-alanine and taurine, etc.
Some of the beta-alanine natural sources are meat, poultry, fish, etc. Some of the commercially popular beta-alanine supplementation brands or products are optimum nutrition beta-alanine, thorne beta-alanine, carnosyn c4, beta-alanine cycling, and beta-alanine caps.
Research is still ongoing to understand the exact role and mechanism by which beta-alanine enhances performance and endurance. In a recent study by Smith CR et al., 2019 they examined the beta-alanine role and found little support for beta-alanine use in resistance exercise performance.
Differences between α-Alanine and β-Alanine |
||
|---|---|---|
| Characteristic Feature | α-Alanine
(alpha-alanine) |
β-Alanine
(beta-alanine) |
| Amino group is attached to … | α-carbon | β-carbon |
| With respect to the occurrence | Relatively more common | Relatively more common/rare |
| IUPAC name | 2-aminopropanoic acid | 3-aminopropanoic acid |
| Stereocenter | Present | Absent |
| Biosynthesis | Via integration of pyruvate & some source of branched-chain amino acids | Via dihydrouracil & carnosine degradation. |
Data Source: Akanksha Saxena of Biology Online
Answer the quiz below to check what you have learned so far about alanine.
References
- Smith, C. R., Harty, P. S., Stecker, R. A., & Kerksick, C. M. (2019). A Pilot Study to Examine the Impact of Beta-Alanine Supplementation on Anaerobic Exercise Performance in Collegiate Rugby Athletes. Sports (Basel, Switzerland), 7(11), 231. https://doi.org/10.3390/sports7110231
- Trifonov EN (December 2000). “Consensus temporal order of amino acids and evolution of the triplet code”. Gene. 261 (1): 139–51. doi:10.1016/S0378-1119(00)00476-5. PMID 11164045.
- “Nomenclature and Symbolism for Amino Acids and Peptides”. IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983.
- Yoshikawa N, Sarower MG, Abe H (2016). “Alanine Racemase and D-Amino Acid Oxidase in Aquatic Animals”. In Yoshimura T, Nishikawa T, Homma H (eds.). D-Amino Acids: Physiology, Metabolism, and Application. Springer Japan. pp. 269–282. ISBN 978-4-431-56077-7.
- Patna BK, Kara TC, Ghosh SN, Dalai AK, eds. (2012). Textbook of Biotechnology. McGraw-Hill Education. ISBN 978-0-07-107007-2.
- Kendall EC, McKenzie BF (1929). “dl-Alanine”. Organic Syntheses. 9: 4. doi:10.15227/orgsyn.009.0004.; Collective Volume, vol. 1, p. 21.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.